Avogadro's number is the number of items in one mole. The number is experimentally determined based on measuring the number of atoms in precisely 12 grams of the carbon-12 isotope, giving a value of approximately 6.022 x 1023.
Cups to Grams Converter Use this calculator tool to convert between cups and grams. Note that because the cup is a unit of volume and gram is a unit of weight, you will need to choose an ingredient from the drop-down list or enter a density figure. For chemistry you often need to convert moles to grams and grams to moles. There is a simple relation between these two:, where - mass of the substance in grams - quantity of the substance in moles - molar mass of the substance in grams/mole. And the most difficult task here is finding out the molar mass.
You can use Avogadro's number in conjunction with atomic mass to convert a number of atoms or molecules into the number of grams. For molecules, you add together the atomic masses of all the atoms in the compound to get the number of grams per mole. Then you use Avogadro's number to set up a relationship between the number of molecules and mass. Here's an example problem that shows the steps:
Avogadro's Number Example Problem
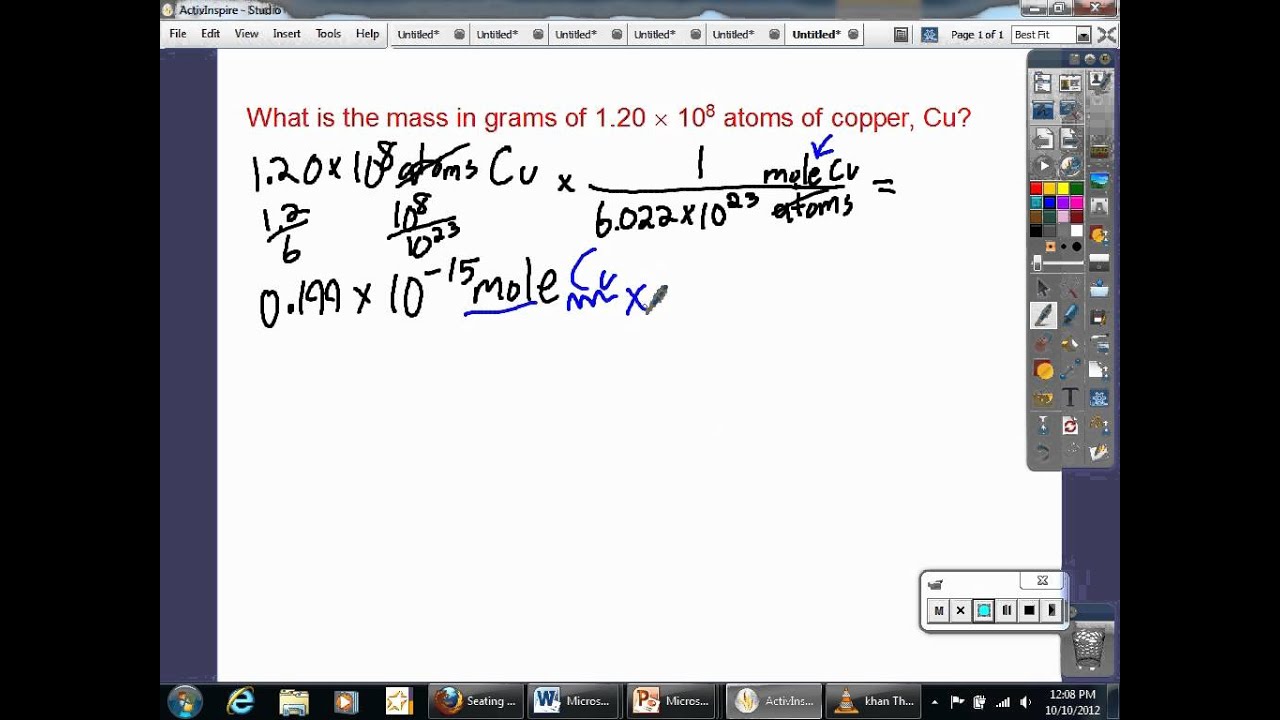
Question: Calculate the mass in grams of 2.5 x 109 H2O molecules.
Solution:
Step 1 - Determine the mass of 1 mole of H2O
To obtain the mass of 1 mole of water, look up the atomic masses for hydrogen and oxygen from the Periodic Table. There are two hydrogen atoms and one oxygen for every H2O molecule, so the mass of H2O is:
Convert Grams To Atoms Worksheet
mass of H2O = 2 (mass of H) + mass of O
mass of H2O = 2 ( 1.01 g ) + 16.00 g
mass of H2O = 2.02 g + 16.00 g
mass of H2O = 18.02 g
Step 2 - Determine the mass of 2.5 x 109 H2O molecules
One mole of H2O is 6.022 x 1023 molecules of H2O (Avogadro's number). This relation is then used to 'convert' a number of H2O molecules to grams by the ratio:
mass of X molecules of H2O / X molecules = mass of a mole of H2O molecules / 6.022 x 1023 molecules
Solve for the mass of X molecules of H2O
mass of X molecules of H2O = ( mass of a mole H2O · X molecules of H2O ) / 6.022 x 1023 H2O molecules
How To Find Atoms From Grams
mass of 2.5 x 109 molecules of H2O = ( 18.02 g · 2.5 x 109) / 6.022 x 1023 H2O molecules
mass of 2.5 x 109 molecules of H2O = ( 4.5 x 1010) / 6.022 x 1023 H2O molecules
mass of 2.5 x 109 molecules of H2O = 7.5 x 10-14 g.
Answer
Convert Atoms To Mass In Grams
The mass of 2.5 x 109 molecules of H2O is 7.5 x 10-14 g.
Helpful Tips for Converting Molecules to Grams
Convert Grams To Atoms Calculator
The key to success for this type of problem is paying attention to the subscripts in a chemical formula. For example, in this problem, there were two atoms of hydrogen and one atom of oxygen. If you're getting the incorrect answer for this type of problem, the usual cause is having the number of atoms wrong. Another common problem is not watching your significant figures, which can throw off your answer in the last decimal place.
Comments are closed.